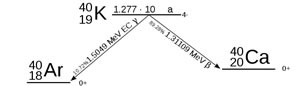
Potassium-40 is an unstable isotope with 40 nucleons (19 protons and 21 neutrons) in the nucleus. It decays by emitting a negative or positive beta particle.
Potassium is an important electrolyte in body fluids and is responsible for the water content in the cells in our bodies. About every 9,000th potassium atom of the approximately 100 grams of potassium in your body is potassium-40.